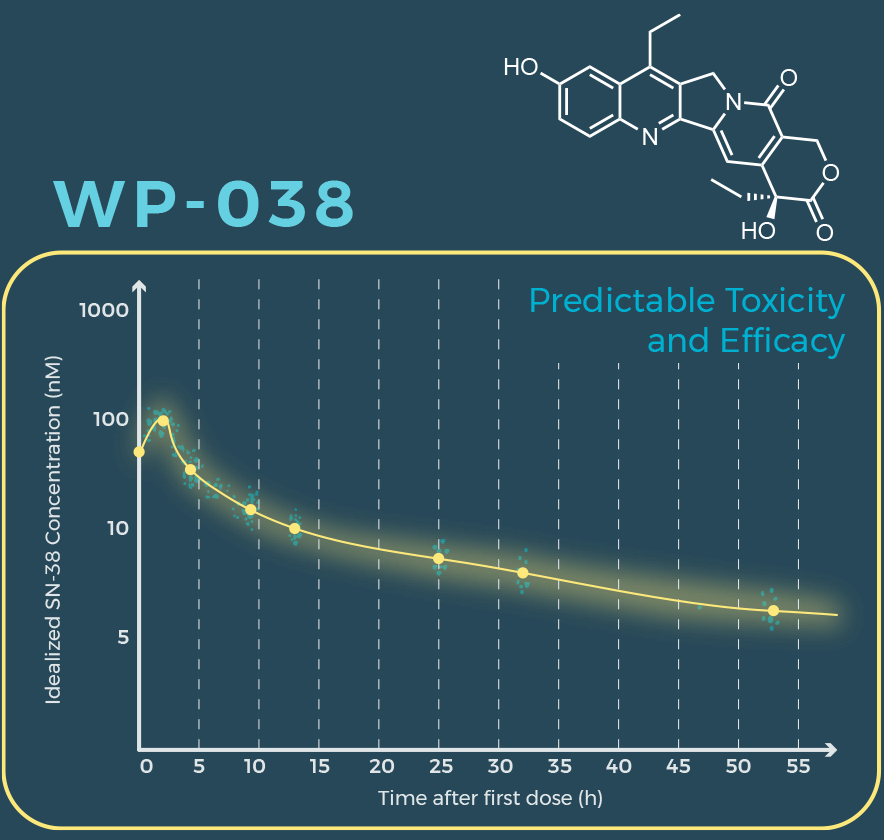
Eliminating irinotecan and dosing WP-038 formulation provides predictable safety and efficacy for the treatment of colorectal and other gastrointestinal cancers.
WP-038
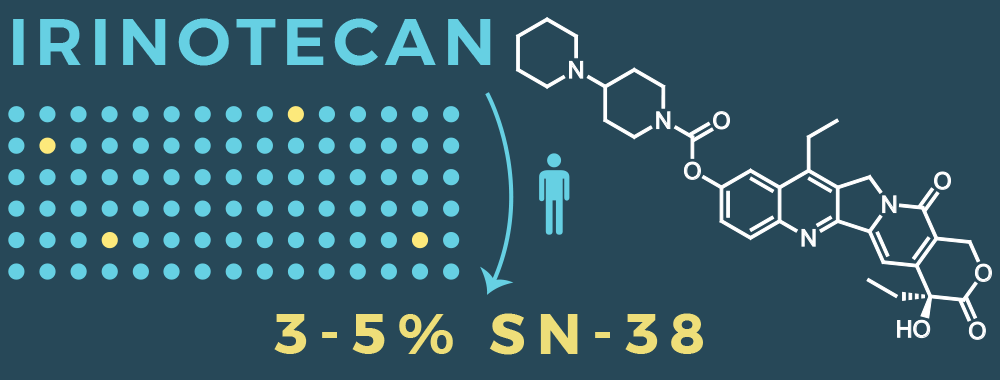
- Irinotecan is metabolized into SN-38, the active anti-cancer agent
- Only 3-5% of irinotecan is converted to active SN-38 in humans
- Unpredictable metabolism results in unpredictable toxicity and efficacy
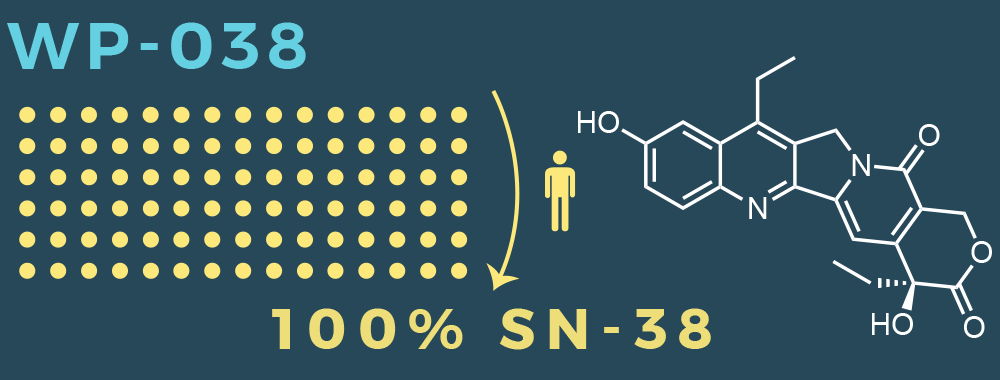
- Apisolex™ excipient allows for the administration of SN-38
- Direct administration of SN-38 will result in better patient outcomes
- Predictable safety and predictable toxicity
100% SN-38
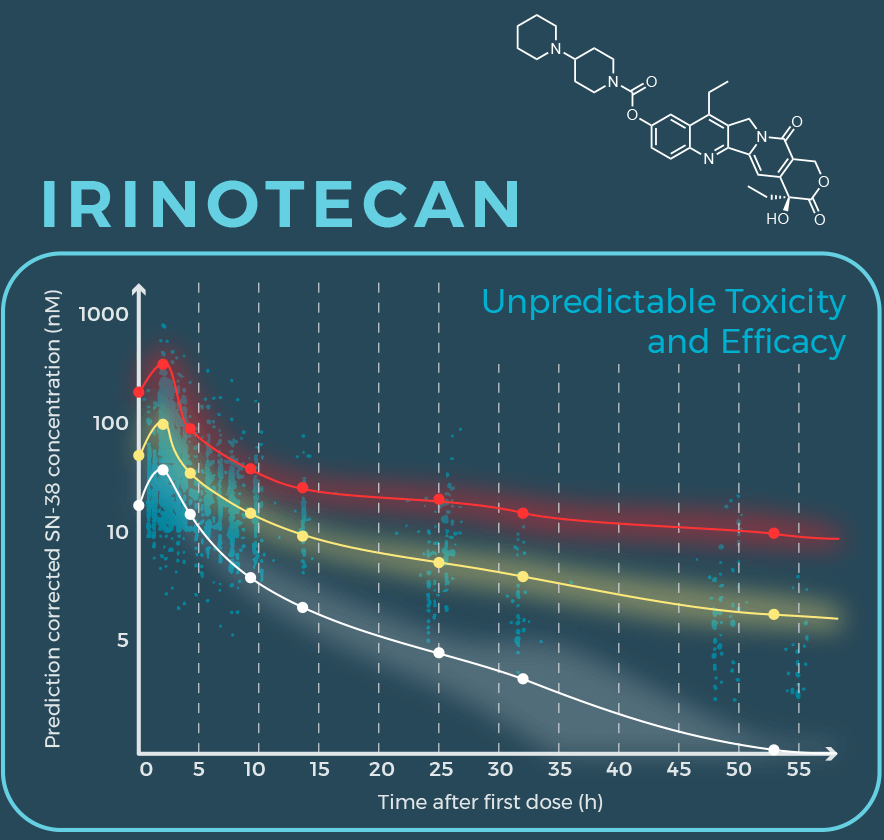
The complex degradation (metabolism) of irinotecan results in highly variable amounts of SN-38 from patient to patient.
Variablity results in many patients with toxic doses (red line) or ineffective doses (white line).
Data reproduced from: The AAPS Journal (2020) 22:59
Eliminating irinotecan and dosing WP-038 formulation provides predictable safety and efficacy.
Predictable toxicity allows for optimal dosing and maximum anti-cancer effectiveness of SN-38.
Optimal dosing of SN-38 is expected to increase patient response rates by ~ 300% over irinotecan